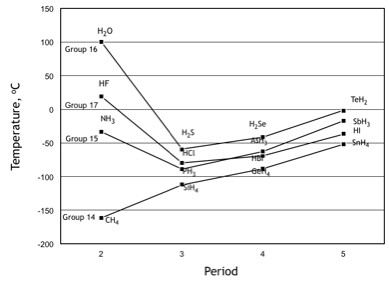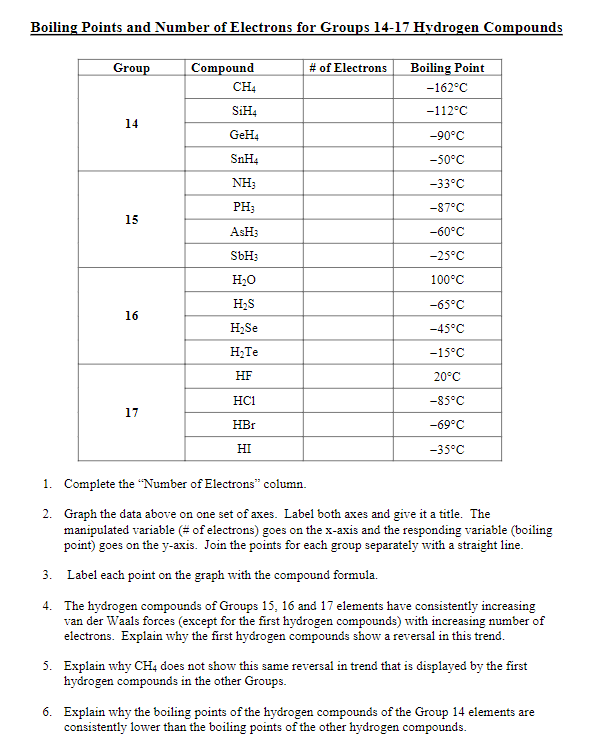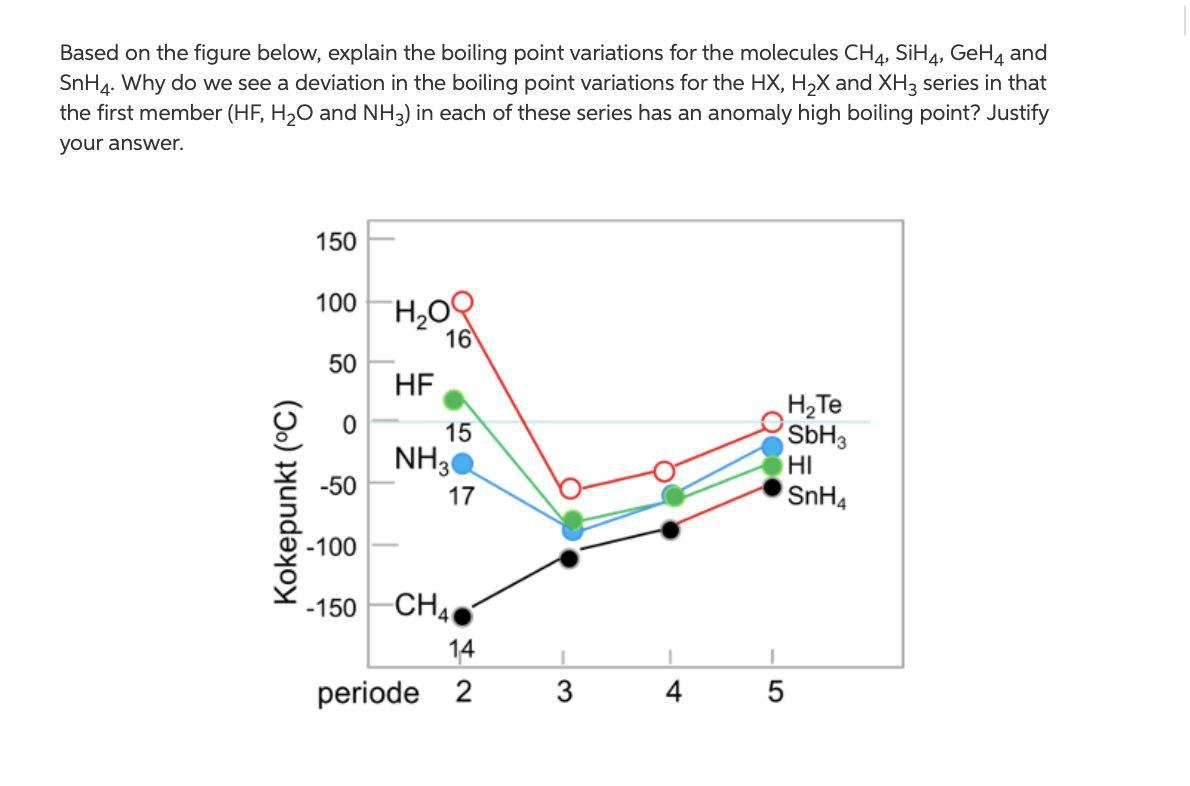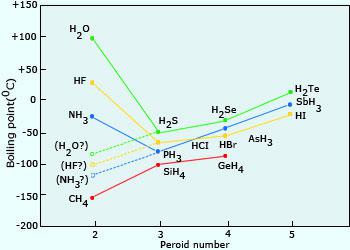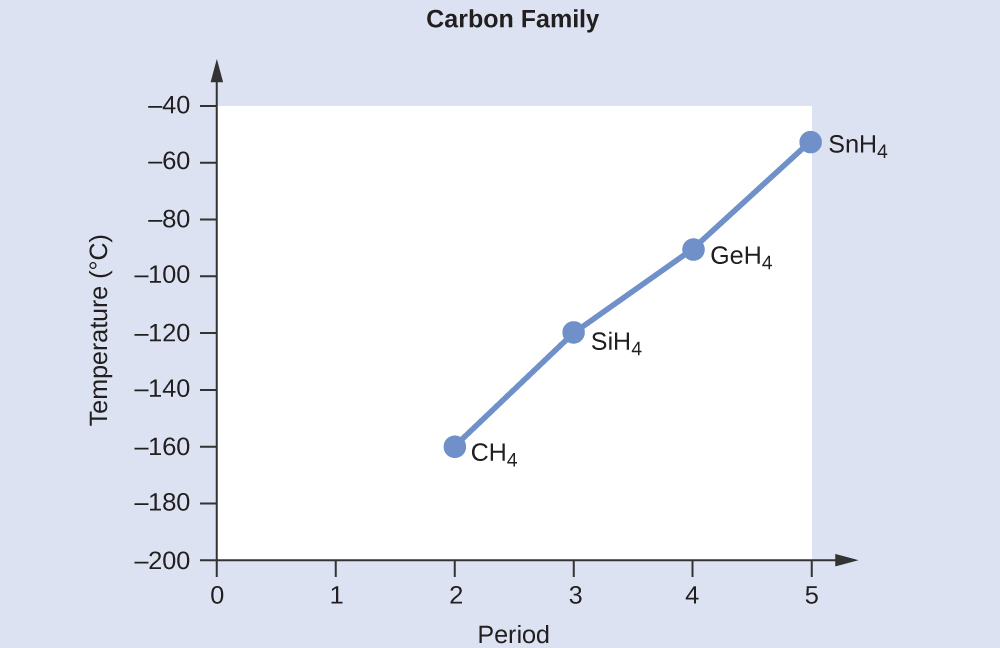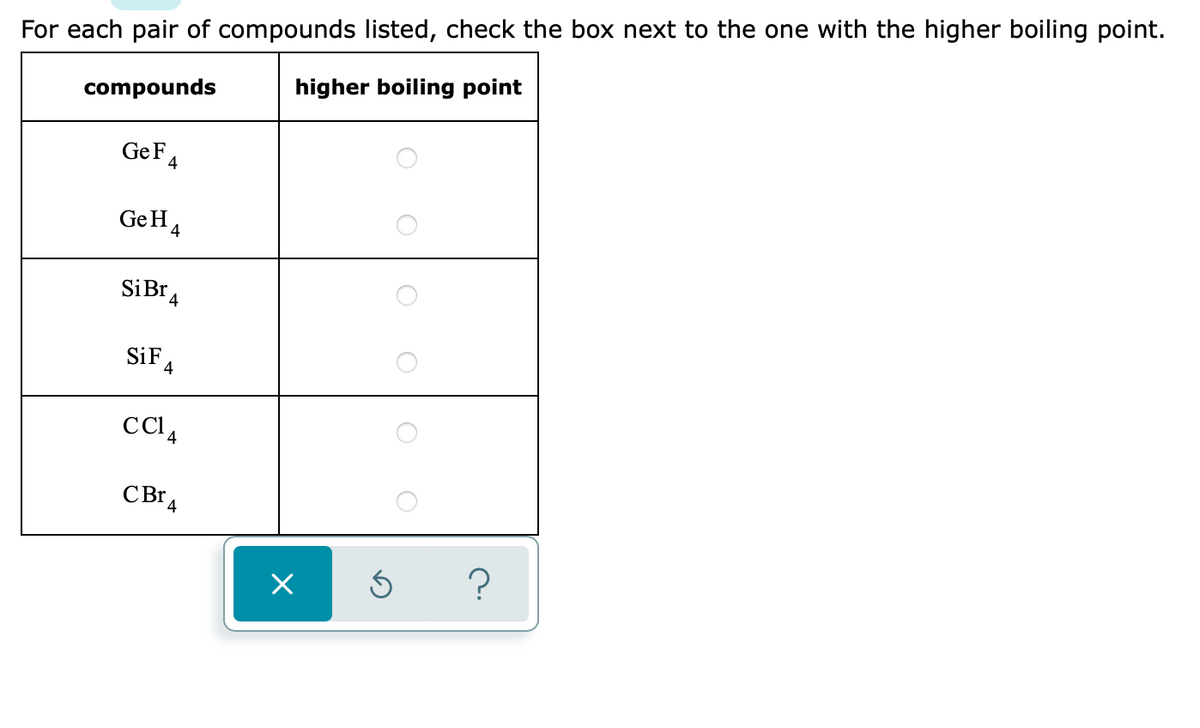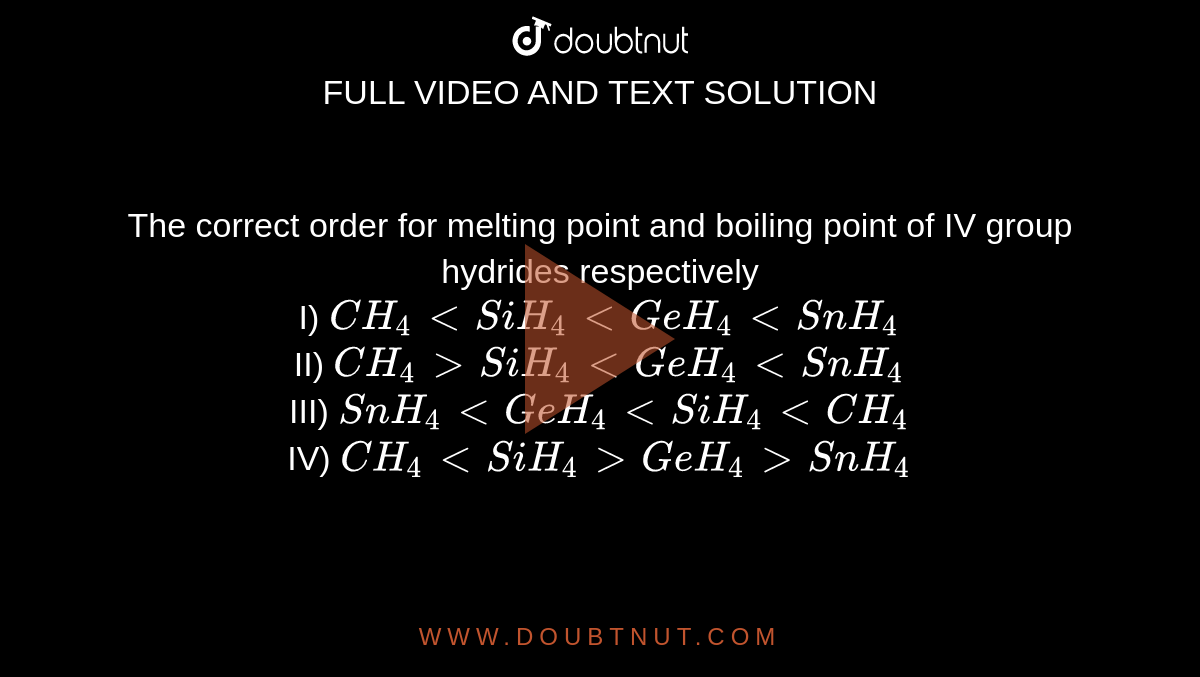
The correct order for melting point and boiling point of IV group hydrides respectively I) CH(4) lt SiH(4) lt GeH(4) lt SnH(4) II) CH(4) gt SiH(4) lt GeH(4) lt SnH(4) III) SnH(4)

SOLVED:Estimate the missing boiling point in the following series of compounds. (a) CH4,-164^∘ C ; SiH4,-112^∘ C ; GeH4,-90^∘ C sn H4, ?^∘ C (b) H2 O, ?^∘ C ; H2 S,-61^∘